Chemical Reaction and Equations class 10 Chapter 1
Chemical Reaction and Equations class 10 taken from NCERT Book Chapter 1 In this article there is full information about the chapter 1 Please read carefully and take solution accouridng to your problems

Chemical Reaction and Equations class 10 what is Chemical Reaction
The Change of one or more substances into other substances having different Composition and different properties is called Chemical Reaction , Example
- Farmation pf curd from milk
- Rusting of Iron
- Burning of wood
- cooking of rice
Consider the following situations of daily life and think what happens when–
- Milk is left at room temperature during summers
- an iron tawa/ pan / nail is left exposed to humid atmosphere
- grapes get fermented
- food is cooked
- food gets digested in our body
- we respire ,
In all the above situations the nature and the identity of the initial substance have somewhat Changed we have already learnt about physical and Chemical Changes of matter in our previous classes whenever a Chemical Change occurs we can say that a Chemical reaction has taken place you may perhaps be wondering as to what is actually meant by a Chemical reaction how do we come to know that a Chemical reaction has taken place? let us perform some activities to find the answer to these questions,
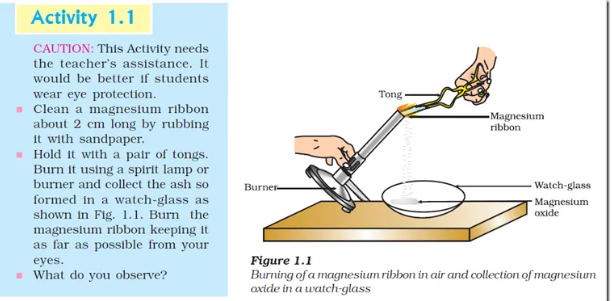
You must have observed that magnesium ribbon burns with a dazzling white flame and Changes into a white power is magnesium oxide it is formed due to the reaction between magnesium and oxygen present in the air,

From the above three activities we can say that any of the following observations us to determine whether a Chemical reaction has taken place-
- Change in state
- Change in colour
- evolution of a gas
- Change in temperature

Chemical Reaction and Equations class 10
As we observe the changes around us,we can see that there is a large variety of Chemical reaction taking place around us we will study about the various types of Chemical reaction and their symbolic representation in this chapter
1.1 Chemical Equation
Activity 1.1 can be described as-when a magnesium ribbon is burnt in oxygen it gets converted to magnesium oxide, this description of a chemical reaction in a sentence form is quite long, it can be written in a shorter form simplest way to do this is write in the form of a word-equation
The word -equation for the above reaction would be —

The substances that undergo chemical the new substance is magnesium and Oxygen are the reaction the new substance is magnesium oxide formed during the reaction as a product A word-equation shows change of reaction to products through an arrow placed between them the reaction are written on the left-hand side[LHS] with a plus sign (+) between them similarly products are written on the right -hand side [RHS] with a plus sing (+) between them the arrowhead points towards the products and shows the direction of the reaction
1.1.1 Writing a chemical Equation
it there any other shorter way for representing chemical equations? chemical equations can be made more concise and useful if we use chemical formulae instead of words A chemical equation represents a chemical reaction if you recall formulae of magnesium oxygen and magnesium oxide the above word-equation can be written as-

Count and compare the number of atoms of each element on the LSH and RSH of the arrow is the number of atoms of each element the same on both the sides?” if yes then the equation is balanced if not then the equation is unbalanced because the mass is not the same on chemical equation for a reaction, Equation (1.2) is a skeletal chemical equation for the burning magnesium in air
1.1.2 balance chemical Equation
Recall the law of conservation of mass that you studied in class IX, mass can neither be created nor destroyed in a chemical reaction that is the total mass of the elements present in the products of a chemical reaction has to be equal to the total mass of the elements present in the reactants In other words the number of atoms of each element remains the same before and after a chemical reaction hence we need to balance a skeletal chemical equation is the chemical Eq, (1.2) balanced ? let us learn about balancing a chemical equation step by step

The above word-Equation may be represented by the following chemical equation –

Let us examine the number of atoms of different elements on both sides of the arrow.
| Element | Number of atoms in reactants (LSH) | Number of atoms in products (RSH) |
| Zn | 1 | 1 |
| H | 2 | 2 |
| S | 1 | 1 |
| O | 4 | 4 |
As the number of atoms of each element is the same on both sides of the arrow Eg (1.3) is a balanced chemical equation
Let us try to balance the following chemical equation

Chemical Reaction and Equations
step: 1 To balance a chemical equation first draw boxes around each formulae Do not change anything inside the boxes while balancing the equation

step:2 List the number of atoms of different element present in the unbalanced equation (1.5)
| Element | Number of atoms in reactants (LHS) | Number of atoms in products (RSH) |
| Fe | 1 | 3 |
| H | 2 | 2 |
| O | 1 | 4 |
step:3 It is often convenient to start balancing with the compound that contains the maximum number of atoms It may be reactant or a product In that compound select the element which has the maximum number of atoms using these Criteria we select Fe3O4 and the element Oxygen In it there are four Oxygen atoms on the RSH and only one on the LSH
To balance the Oxygen atoms–
| Atoms of Oxygen | In Reactants | In products |
| (1) Initial | 1 (in H2O) | 4(in Fe3O4) |
| (2) To balance | 1 X 4 | 4 |
To Equalise the number of atoms it must be remembered that we cannot alter the formulae of the compounds or elements involved in the reactions for example” to balance Oxygen atoms we can put coefficient 4 as 4 H2 O and not H2 O4 or (H2O)4 Now the party balanced equation becomes-

Step:4 Fe and H atoms are still not balanced pick any of these element to proceed further let us balance Hydrogen atoms in the party balanced equation
To equalise the number of H atoms make the number of molecules of hydrogen four on the RSH .
| Atoms of hydrogen | In reactants | In products |
| (i) Initial | 8(in 4 H2O) | 2(In H2) |
| (ii) To balance | 8 | 2 X 4 |

step:5 Examine the above equation and pick up the third element which is not balanced you find that only one element is left to be balanced that is Iron
| Atoms of Iron | In reactants | In products |
| (i) Initial | 1 (In Fe) | 3 (in Fe3O4) |
| (ii) To balance | 1 X3 | 3 |
To Equalise Fe, we take three atoms of Fe on the LSH

Step:6 Finally to check the correctness of the balanced equation we count atoms of each element on both sides of the equation

The number of atoms of element on both sides of Eq (1.9) are equal this equation is now balanced this method of balancing chemical equation is called hit-and trial method as we make trails to balance the equation by using the smallest whole number coefficient
step:7 Writing symbols of physical states, carefully examine the above balanced Eq (1.9) Does this equation tells us anything about the physical state of each reactant and product? no information has been give in this equation about their physical states
To make a chemical equation more informative the physical states of the reactant and product are mentioned along with their chemical formulae the gasecous liquid aqueous and solid states of reactant and products are represented by the notation (g) (I) (Eq) and (s) respectively the word aqueous (aq) is written if the reactant or product is present as a solution in water
Chemical Reaction and Equations class 10
The balanced Eq. (1.9) becomes

Note that the symbol (g) is used with H2O to indicate that in this reaction water is used in the form of stem Usually physical states are not included in a chemical equation unless it is necessary to specify them sometimes the reaction condition such as temperature pressure catalyst etc for the reaction are indicated above and / or below arrow in the equation for example,

Using these steps can you balance Eq (1.2) give in the text earlier?
Chemical Reaction and Equations class 10
1.2 Types of Chemical Reactions
We have Learnt in class IX that during a chemical reaction atoms of one element do not change into those of another element Nor do atoms disappear from the Mixture or appear from elsewhere Actually chemical reaction invoice that breaking and making of bonds between atoms to produce new substances you will study about types of bonds formed between atoms in Chapter 3 and 4
1.2.1 Combination Reaction

In this reaction calcium oxide and water combine to form a single product calcium hydroxide such a reaction In which a single product is formed from two or more reactants is known as a combination reaction ,
Let us discuss some more examples of combination reaction
(i) Burning of coal

(ii) Formation of water from H2 (g) and O2 (g)

In simple language we can say that when two or more substances ( element or Compounds ) Combine to form a single product the reactions are called Combination reaction
In Activity 1’4 we also observed that a large amount of heats is evolved this makes the reaction mixture warm Reaction in which heart is released along with the formation of products are called Exothermic Chemical reaction
Other examples of exothermic reactions are—
(i) Burning of natural gas

(ii) Do you know that respiration is an exothermic process?
We all know that we need energy to stay alive we get this energy from the food we eat during digestion food is broken down into simpler substances for example rice potatoes and bread contain carbohydrates these carbohydrates are broken down to form glucose this glucose combines with Oxygen in the cells of our body and provides energy the special name of this reaction is respiration the process of which you will study in chapter 6

(iii) The decomposition of vegetable matter into compost is also an example of an exothermic reaction identity the type of the reaction taking place in Activity 1.1 where heat is given out along with the formation of a single product ,
Chemical Reaction and Equations class 10
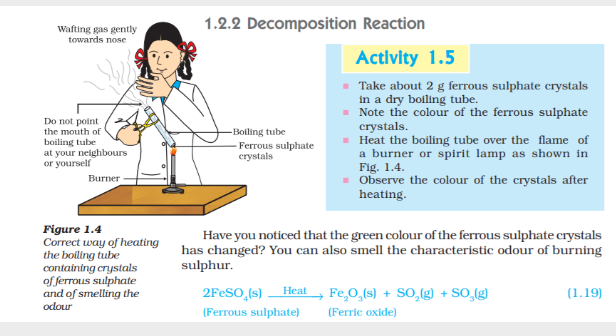
In this reaction you can observe that a single reactant breaks down to give simpler products this is a decomposition reaction ferrous Sulphate Crystals (FeSO4 7H2O) lose water when heated and the Colour of the Crystals Changes it then decomposes to ferric Oxide (Fe2O3) Sulphur Dioxide (SO2) and sulphur trioxide (SO3) ferric oxide is a solid while SO2 are gases
Decomposition of calcium carbonate to calcium oxide and carbon dioxide on heating is an important decomposition reaction used in various industries calcium oxide is called lime or quick lime it has many uses—One is in the manufacture of cement when a decomposition reaction is carried out by heating it is called thermal decomposition
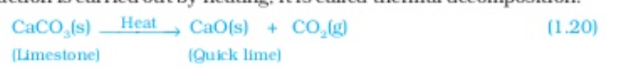
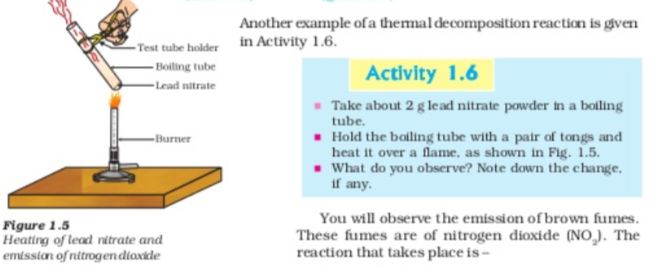
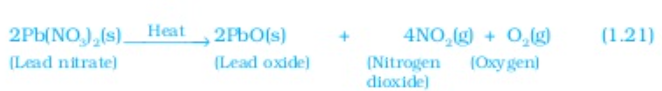
Let us perform some more decomposition reaction as given in activity 1.7 and 1.8
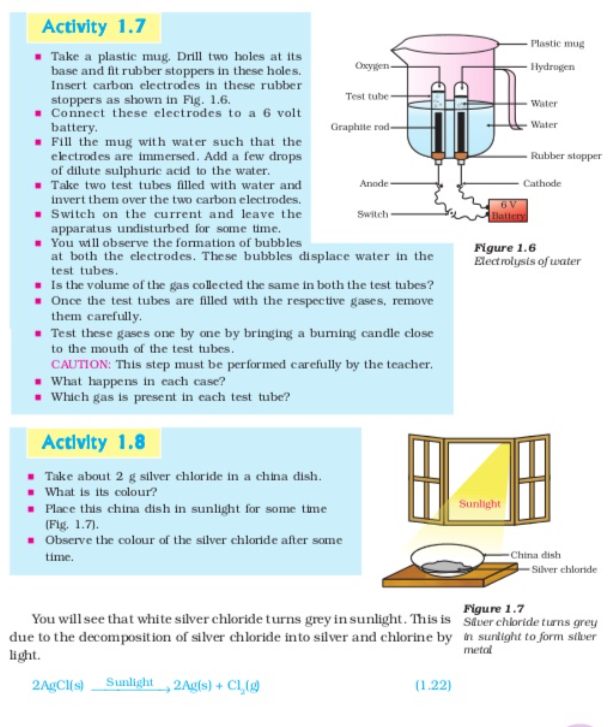
Silver Bromide also behaves in same way
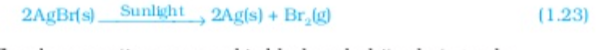
The above reactions are used in black and white photography what form of energy is causing these decomposition reactions? we have seen that the decomposition reactions requite energy either in the form of heat light or electricity for breaking down the reactants Reaction in which energy is absorbed are known as endothermic reactions,


In this reaction Iron has displaced or removed another element copper from copper sulphate solution this reaction is known as displacement reactions, other examples of displacement reactions are
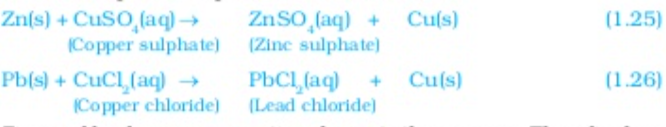
Zinc and lead are more reactive elements than copper, they displace copper from it compounds

what causes that? the white precipitate of BaSO4 is formed by the reaction of of SO2-4 and Ba2+ the other product formed is sodium chloride which remains in the solution such reaction in which there is an exchange of irons between the reaction are called double displacement reaction ,
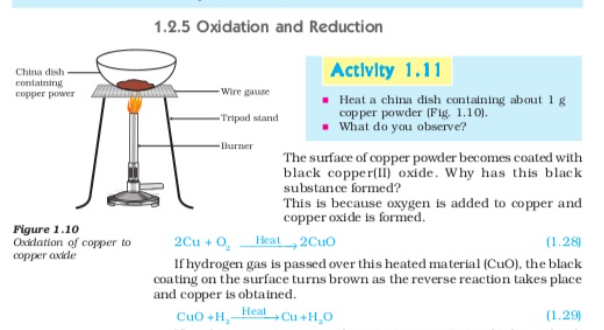
if a substance gains oxygen during a reaction it is said to be Oxidised if a a substance loses oxygen during a reaction it is said to be reduced during this reaction (1.29) the copper (II) Oxide is losing oxygen and is being reduced the hydrogen is gaining oxygen and is being oxidised In other words one reactant gets oxidised while the other gets reduced during a reaction such reactions are called Oxidation-reduction reactions or redox reactions,
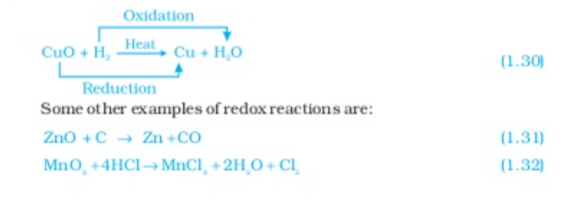
In reaction (1.31) carbon is Oxidised to CO and ZnO is reduced to Zn In reaction (1.32) HCI is Oxidised to CI2 whareas MnO2 is reduced to MnCI2 from the above examples we can say that if a substance gains Oxygen or loss hydrogen during a reaction it is oxidised if a substance loses oxygen or gains hydrogen during a reaction it is reduced
Thanks for sharing. I read many of your blog posts, cool, your blog is very good.
thanks